Immune and non-immune functions of Tregs in human tissues
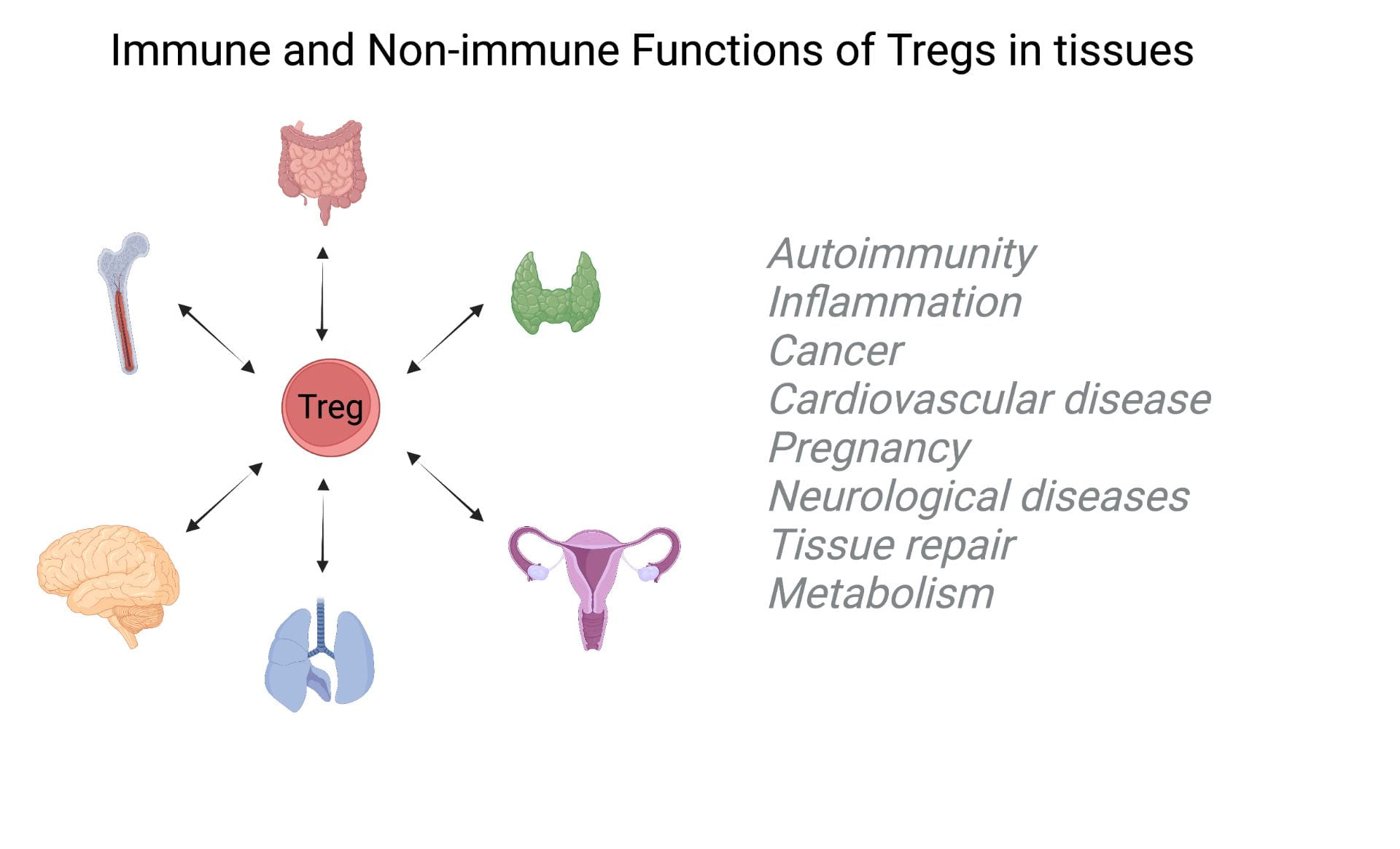
In an arms race against pathogens, the adaptive immune system has evolved as a complex cellular and molecular machinery capable of generating and selecting targeted responses to specific antigens. To prevent autoimmunity, i.e., maintain tissue tolerance, lymphocytes recognizing tissue antigens during B and T cell ontogeny are deleted (central tolerance). At the periphery, regulatory T cells, a subset of CD4+ T cells, complement the process by actively suppressing autoreactive T cells in tissues. Beyond their role in tolerance, Treg cells were found to have unexpected roles in maintaining homeostatic tissue functions. They influence insulin sensitivity in adipose tissue and support stem cell niches in the skin, gut, and bone marrow. We study immune and non-immune functions of Tregs in human tissues (and mouse models) using systems biology approaches (single-cell genomics, spatial transcriptomic, multiplexed imaging). We also develop new approaches to test their functions (e.g. antigen recognition, and suppression).
Zemmour D, Charbonnier LM, Leon J, Six E, Keles S, Delville M, Benamar M, Baris S, Zuber J, Chen K, Neven B, Garcia-Lloret MI, Ruemmele FM, Brugnara C, Cerf-Bensussan N, Rieux-Laucat F, Cavazzana M, André I, Chatila TA, Mathis D, Benoist C. Single-cell analysis of FOXP3 deficiencies in humans and mice unmasks intrinsic and extrinsic CD4 + T cell perturbations. Nature Immunology. 2021 Apr 8;1–13. PMID: 33833438
Zemmour D, Zilionis R, Kiner E, Klein AM, Mathis D, Benoist C. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nature Immunology. 2018 Feb 12. PMID: 29434354
DiSpirito JR*, Zemmour D*, Ramanan D, Cho J, Zilionis R, Klein AM, Benoist C, Mathis D. Molecular diversification of regulatory T cells in nonlymphoid tissues. Science Immunology. 2018 Sep 14;3(27):eaat5861. PMID: 30217811
Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, Mathis D. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell. 2018 May 24; PMID: 29887374
Zemmour D, Pratama A, Loughhead SM, Mathis D, Benoist C. Flicr, a long noncoding RNA, modulates Foxp3 expression and autoimmunity. Proc Natl Acad Sci U S A. 2017 Apr 10; PMID: 28396406
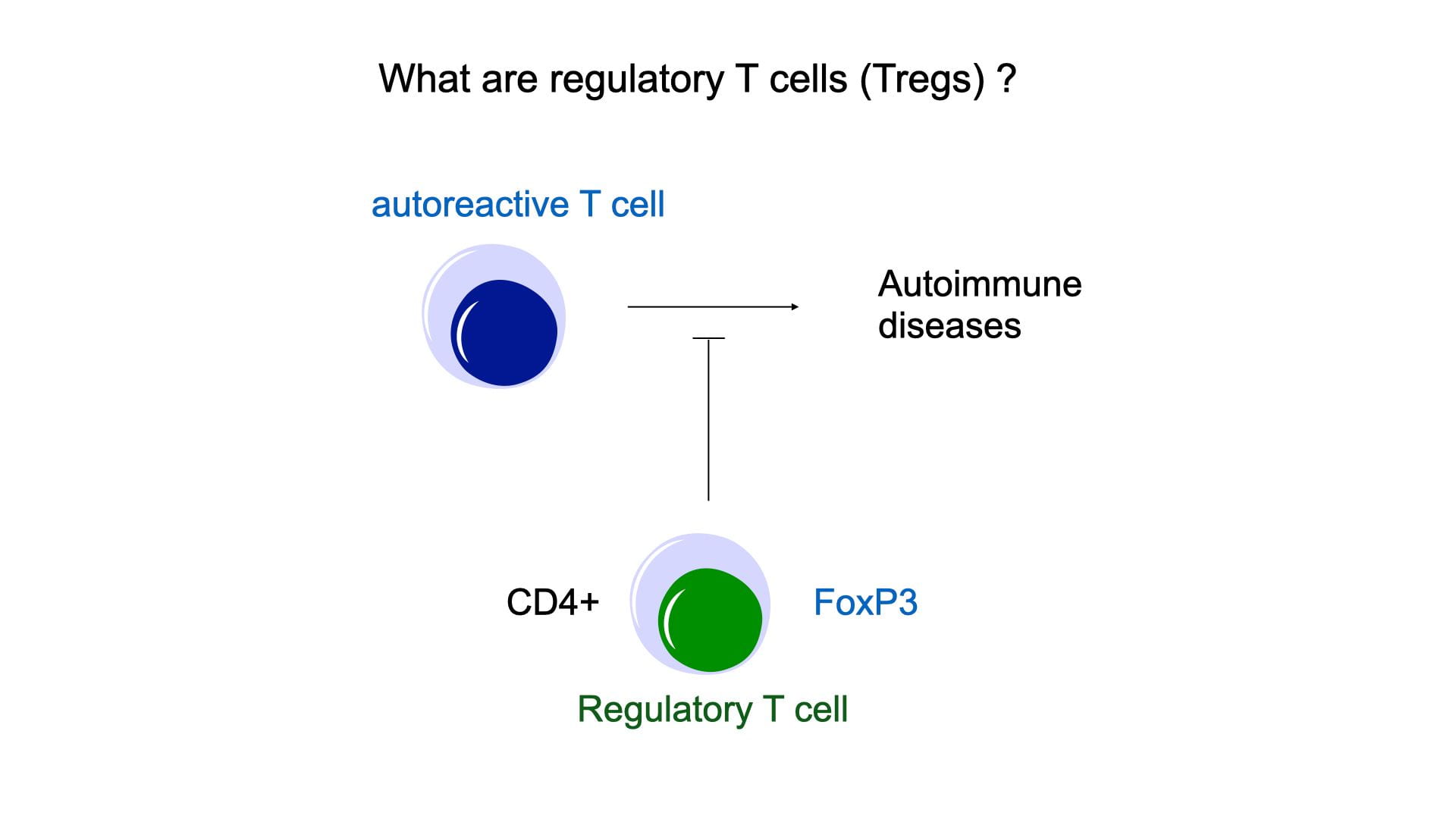
The model: Treg deficiencies
Treg deficiencies offer unique opportunities to dissect the functions of Tregs in both humans (IPEX syndrome) and mice (scurfy). In fact, the mapping of a rare mendelian disease named IPEX (Immune dysregulation Polyendocrinopathy X-linked) to the transcription factor FoxP3 led to the discovery of Tregs in the early 2000s. FoxP3 was demonstrated to be specifically expressed in Tregs. And transgenic mouse models targeting FoxP3 (e.g. Foxp3-GFP for visualization, FoxP3-CRE for Treg-specific knock-downs) spurred numerous studies about the cellular and molecular characterization of Tregs. But these studies were only possible in mice, and our current understanding of Tregs in humans lags behind, particularly in tissues. In the lab, we use single-cell genomics and systems biology to study Treg deficiencies in humans and use mouse models for further experimentations. By contrasting with healthy donors (and WT mice), we uncover the physiological role of Tregs in maintaining tolerance and tissue homeostasis.
We hypothesize that Treg deficiencies can also be more subtle and common and responsible for other autoimmune conditions. The lab focuses on immune checkpoint inhibitor (ICI)-induced autoimmunity, which represents a significant clinical challenge and an opportunity to study the onset of autoimmunity in humans. Indeed, these new drugs have revolutionized cancer treatment, a discovery recognized by the Nobel Prize in Medicine in 2018 to Drs. James P. Allison and Tasuku Honjo. But they also trigger autoimmunity in up to 40% of patients, which can be fatal or result in stopping a potentially curative treatment. Although the cause of the disease is clear (drug-induced), the exact process leading to the pathology is still unknown. We are investigating why Tregs aren’t sufficient to prevent ICI autoimmune disease and ways to manipulate them to prevent autoimmunity while maintaining anti-tumor immunity, a major objective of tumor immunotherapy.
Zemmour D, Charbonnier LM, Leon J, Six E, Keles S, Delville M, Benamar M, Baris S, Zuber J, Chen K, Neven B, Garcia-Lloret MI, Ruemmele FM, Brugnara C, Cerf-Bensussan N, Rieux-Laucat F, Cavazzana M, André I, Chatila TA, Mathis D, Benoist C. Single-cell analysis of FOXP3 deficiencies in humans and mice unmasks intrinsic and extrinsic CD4 + T cell perturbations. Nature Immunology. 2021 Apr 8;1–13. PMID: 33833438
Treg Cellular Interactions Networks
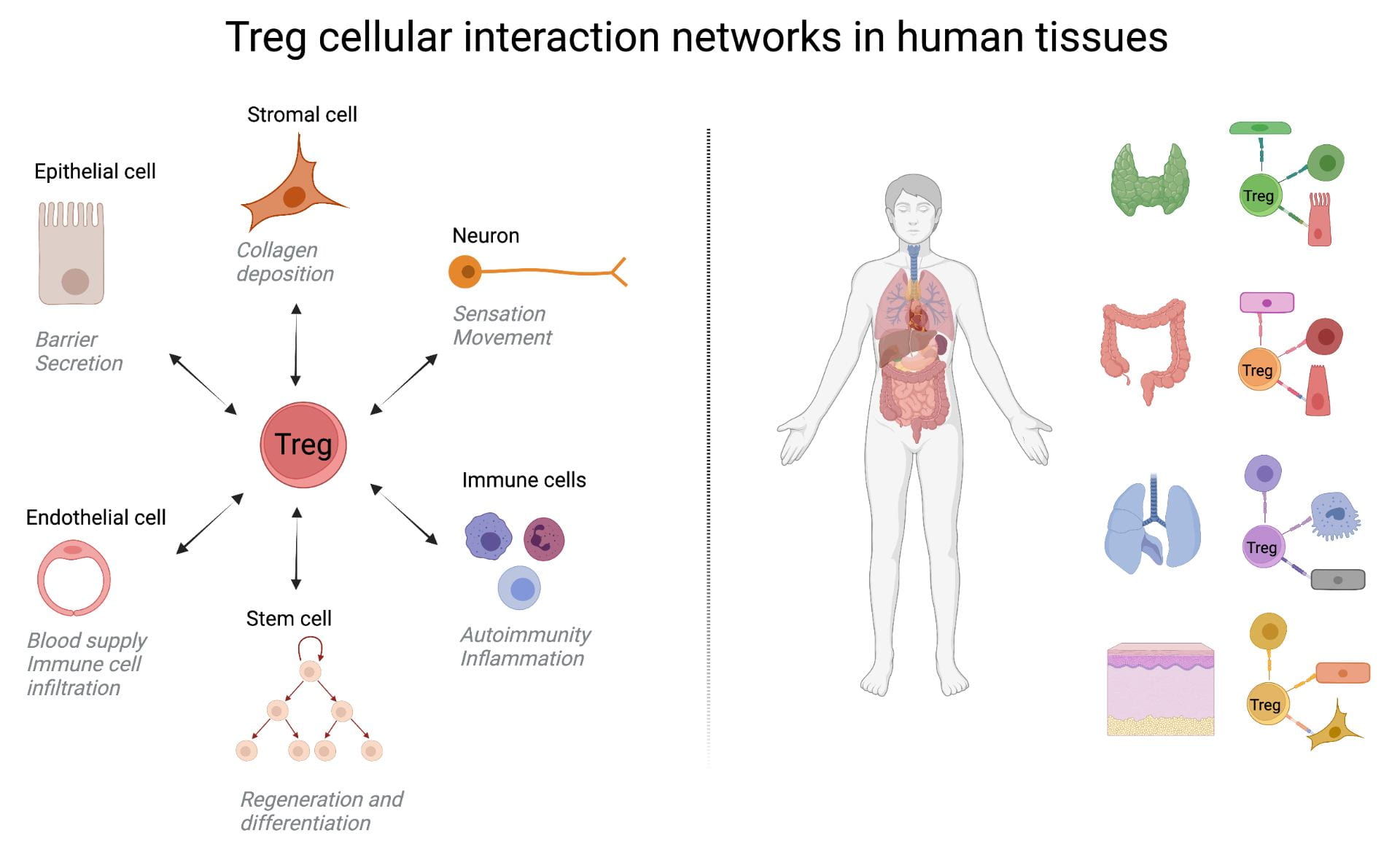
Efforts in specific lab-based projects and larger consortiums like the Human Cell Atlas are mostly concentrated on mapping all cell types in the human body. But we are not single-cell organisms! Understanding immune responses, tissue organization, and bodily functions requires understanding how cells talk to each other. What are the different modes of communication? Are there independent channels? New channels? Are all cells able to talk to each other? We believe that a systematic analysis of cellular interactions will create a new field in biology with an impact on diagnosis and treatments. Many current medications are already targeting cellular interactions (e.g. immune checkpoint blockages, biotherapies in autoimmunity) but none directly measure the impact on cell communications per se. Methods are largely lacking. In the lab, we invent and use new methods from Computational Biology (modeling), Systems Biology, and Synthetic Biology to characterize and modulate these cell communication in vivo and in situ in human tissues. Focusing on Treg cells because of their fundamental importance, we dissect their network of cellular interactions across tissues and diseases.
Wang K, Yaghi OK, Spallanzani RG, Chen X, Zemmour D, Lai N, Chiu IM, Benoist C, Mathis D. Neuronal, stromal, and T-regulatory cell crosstalk in murine skeletal muscle. Proc Natl Acad Sci U S A. 2020 Mar 10;117(10):5402–5408. PMID: 32102913
Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, Mathis D. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Science Immunology. 2019 May 3;4(35). PMID: 31053654
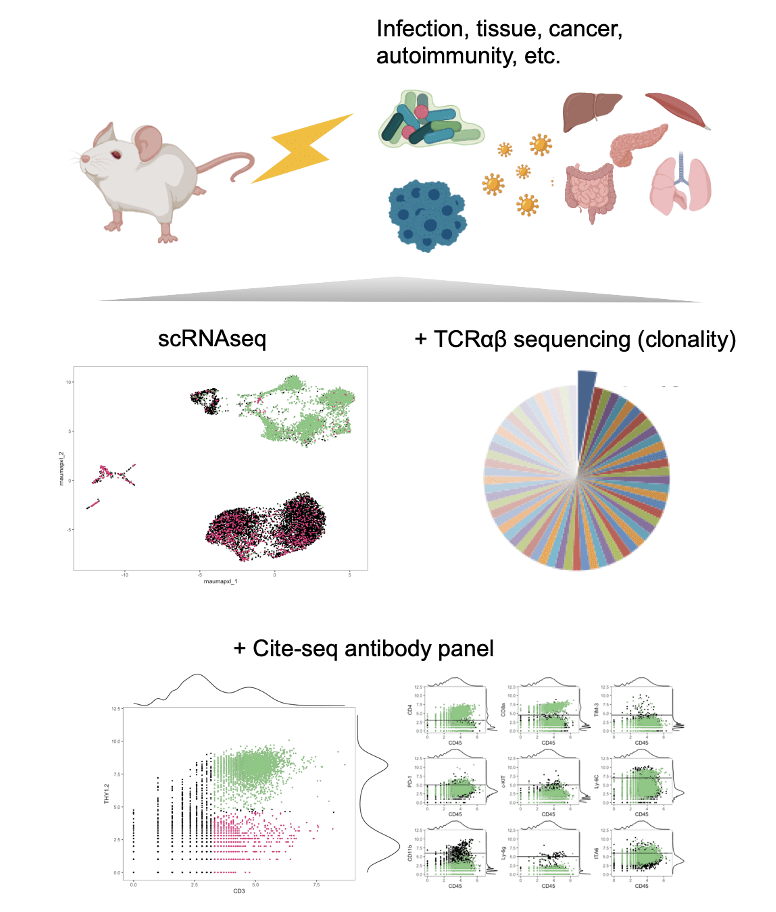
What about the other T cells? ImmGen T, a Mouse T cell atlas
T cells are very heterogeneous and versatile across tissues and pathologies (e.g. infections, cancer, autoimmunity). The old classification of T cells only scratched the surface of a world being revealed by single-cell genomics. We are part of a consortium aiming to characterize T cells in all their shapes and flavors in mice: Immgen T. ImmGen T is an Open Source Project part of the ImmGen consortium. We look where and when they matter the most, beyond healthy tissues, e.g. infections, autoimmunity, aging, and cancer (see landscape).
Zemmour D, Goldrath A, Kronenberg M, Kang J, Benoist C. The ImmGen consortium OpenSource T cell project. Nature Immunology. 2022 May;23(5):643–644. PMID: 35469020
Zemmour D, Kiner E, Benoist C. CD4+ teff cell heterogeneity: the perspective from single-cell transcriptomics. Curr Opin Immunol. 2020 Apr;63:61–67. PMID: 32259715
Hematopathology: Human T cells in the Clinic
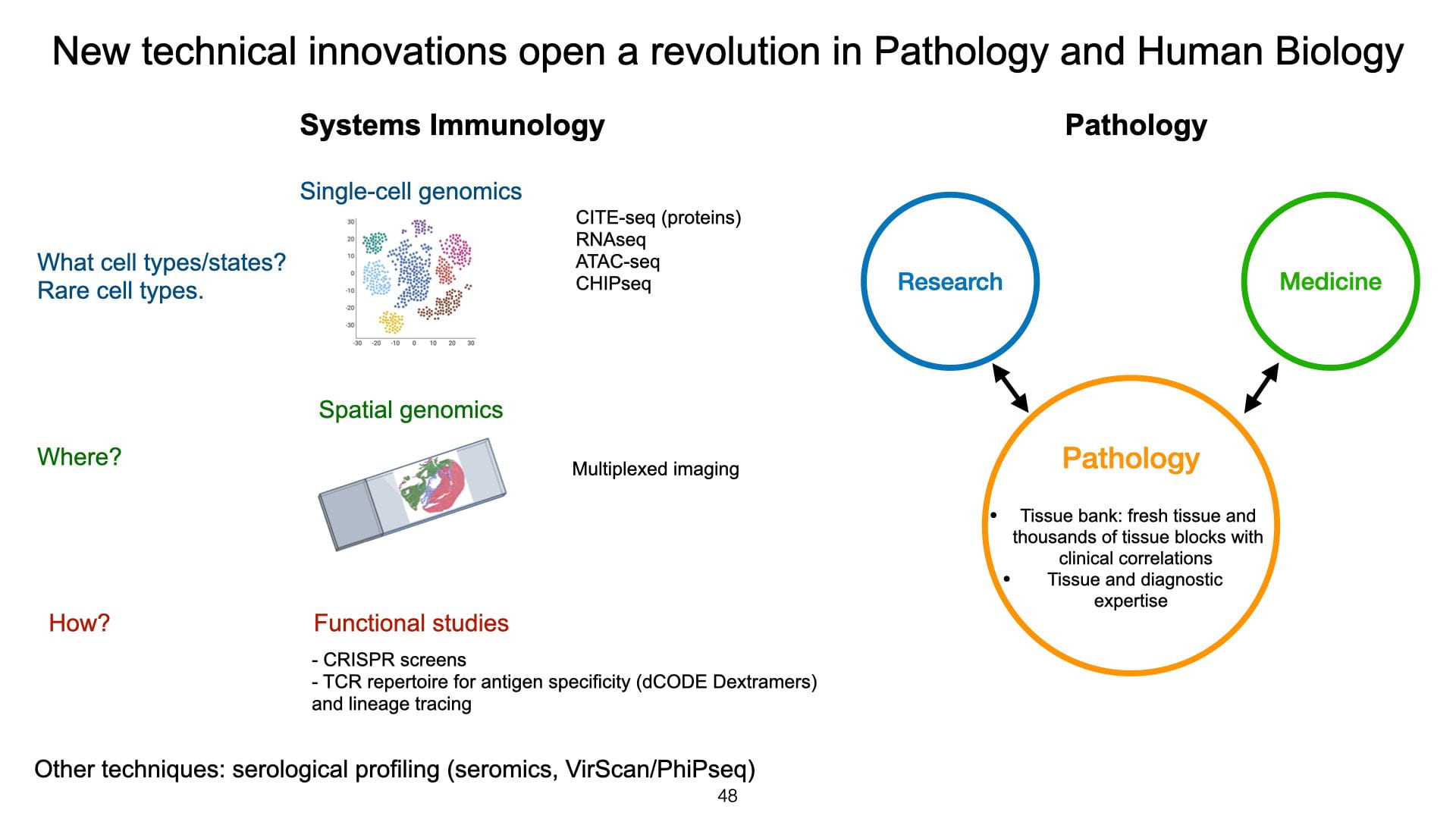
The discrepancy between our knowledge of T-cell biology and its clinical application is striking. And to this day, there is no comprehensive clinical testing of our immune system. Most clinical decisions are based on total lymphocyte counts in a “CBC with differential” (“Complete Blood Count”), rarely flow cytometry (HIV diagnosis, T cell neoplasms). But we know that the immune system is involved in a wide range of diseases: infections, autoimmunity, cancer, cardiovascular, metabolic, and neurological disease. In the lab, we characterize the states of T cells using systems biology approaches (single-cell genomics, spatial transcriptomics) directly in human tissues. And we develop new technologies to detect and assess their functions (e.g. TCR clonality, epitope recognition, cytokine secretion). We are also particularly interested in T-cell neoplasms. They are rare neoplasms notoriously difficult to diagnose because they are heterogeneous diseases that can overlap with non-neoplastic (reactive) T-cell populations both clinically and morphologically. Using single-cell genomics, we redefine the states and normal cellular components of these cancers (e.g. Tregs, Tfh, Th1).
Zemmour D, Kim A. Angioimmunoblastic T cell lymphoma with RHOA mutation. Book Chapter in Molecular Hematopathology-An Integrated Case-Based Approach. 2021. Authors: Rose C Beck, Erika M Moore. ISBN: 978-0-89189-6814
